Electric Charges and Field
WHAT IS ELECTRIC CHARGE ?
- Matter is made of elementary particles, including electrons, protons, and neutrons.
- These particles attract each other through gravitational forces based on their mass.
- However, electrons and protons also experience an electric force, which is much stronger than the gravitational force between them.
- This electric force is due to the property of electric charge, which is an additional property of particles apart from their mass.
- Electrons and protons have charge, while neutrons do not.
- Two electrons placed 1 cm apart from each other repel each other with a force of 2.3 × 10 – 24 N, due to their electric charge.
- Two protons placed 1 cm apart from each other also repel each other with a force of 2.3 × 10 – 24 N, also due to their electric charge.
- Two neutrons placed 1 cm apart from each other attract each other with a very weak force of 1.9 × 10 – 60 N, due to their gravitational force. They do not experience any electric force as they have no charge.
Two Kinds of Charges
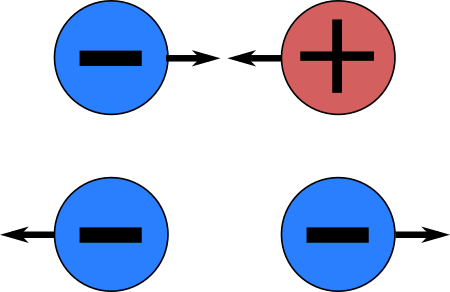
- There are two kinds of charges: positive and negative.
- The electric force between two electrons is the same as the electric force between two protons placed at the same separation.
- The amount of charge on an electron is the same as that on a proton.
- A proton and an electron attract each other with an electric force of 2.3 ×10 – 24 N when placed 1 cm apart.
- The charge on an electron repels the charge on another electron but attracts the charge on a proton.
- The charge on an electron and that on a proton have the same strength but are of two different natures.
- If we pack a proton and an electron together in a small volume, the combination does not attract or repel another electron or proton placed at a distance.
- The net charge on the proton-electron system seems to be zero.
- To differentiate between positive and negative charges, we arbitrarily call the charge on a proton as positive and that on an electron as negative.
- This assignment of positive and negative signs to the proton charge and the electron charge is purely a convention.
- This convention does not mean that the charge on an electron is “less” than the charge on a proton.
Unit of Charge
- Charge is a basic property associated with elementary particles.
- Its definition is as difficult as the definition of mass, time, or length.
- Charge can be measured by comparing it with the charge on a standard body, but we do not know what exactly it is that we intend to measure.
- The SI unit of charge is coulomb, abbreviated as C.
- One coulomb is the charge flowing through a wire in 1 second if the electric current in it is 1 ampere (1 A).
- The charge on a proton is e = 1.60218 ×10 – 19 C.
- The charge on an electron is the negative of the value of the charge on a proton.
Charge is Quantized
- All observable charges in the universe must be integral multiples of the charge on a single proton or electron.
- The net charge on an object containing n1 protons and n2 electrons is (n1 – n2)e, where e is the charge on a single proton or electron.
- Other elementary particles besides protons and electrons also carry charges, but they are also integral multiples of e.
- Charge on any object is always an integral multiple of e and can only be changed in steps of e.
- Charge is quantized.
- The step size of e is usually very small compared to the charges typically found in everyday life.
- In many cases, we can assume a continuous charge variation and neglect the quantization.
Charge is Conserved
- The charge of an isolated system is conserved, meaning it cannot be created or destroyed.
- Charged particles can be created or destroyed, but the net charge of a system remains constant.
- In a beta decay process, a neutron converts into a proton and a new electron is created.
- Before and after the event, the net charge remains zero, as the proton and electron carry equal and opposite charges.
Different methods of Charging
(I) Frictional Electricity / Charging by Friction :
“The electricity developed by rubbing or friction is called frictional electricity.”
- The electrons of the outer shell of an atom is loosely bound to the nucleus. So, when two materials are rubbed against each other, electrons are transferred from one material to other.
- For example, when a glass rod is rubbed with a silk cloth. Some electrons are transferred from the glass rod to the silk cloth. So the glass rod develops a positive charge due to deficiency of electrons while the silk cloth develops an equal negative charge due to excess of electrons.
- Energy required to remove an electron from the surface of a material is called its Work Function. When two different bodies are rubbed, electrons are transferred from a material with lower work function to a material with higher work function.
- Historically the credit of discovery of the fact that amber rubbed with wool or silk cloth attracts light objects goes to Thales of Miletus, Greece, around 600 BC. The name electricity is coined from the Greek word ‘elektron’ meaning ‘amber’.
-
(II) Electrostatic Induction / Charging by Induction :
“It is process of charging an uncharged body with the help of a charged body without touching them with other.”
qin = –q (1–1/ɛr) (where, ɛr is the Dielectric constant of material of the neutral body)
- Let a +vely charged body ‘A’ is taken near an uncharged body (conductor) ‘B’ as shown in the figure.
- –ve charges are attracted & come together at the near end of ‘B’. So –ve charge is induced at the near end & +ve charge (due to deficiency of electrons) is induced at the far end of ‘B’.
- Now the far end of ‘B’ is grounded. So that all the +ve charges in it will be neutralized by the flow of electrons from the ground.
- Now only –ve charges remained in the conductor ‘B’ even after the removal of the charged object ‘A’.
- Note: Magnitude of induced charge (qin) can be lesser or equal to that of inducing charge (q), but it never greater than inducing charge.
(III) Charging by Conduction :
“ “It is the process of charging in which one charged object is touched with another charged or uncharged object & the total charge is shared among them.”
- If the objects are identical, then total charge is equally shared among them.
If the objects are not identical, then charge flows from one body to another till the bodies acquire same potential.
PERMITIVITY OF A MEDIUM:
- “Permittivity (ε) is a property of the medium which determines the electric force between two charges situated in that medium.”
- “Relative permittivity / Dielectric constant of a medium is defined as the ratio of permittivity (ε) of the medium to the permittivity of the free space (ε0 ).”
![]()
- The value of εr for vacuum / free space = 1, for air = 1.00054
- for water = 80, for a conductor εr = & for an insulator (εr > 1)
- “The relative permittivity, of any medium can also be defined as the ratio of the force between two charges kept in vacuum to the fore between them kept in the given medium.”
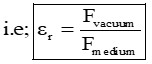
COULOMB’S LAW OF ELECTRIC FORCES :
It states that, “the force of attraction or repulsion acts along the line joining two stationary point charges is directly proportional to the product of the magnitudes of the two charges & inversely proportional to the square of the distance between them.”
![]()
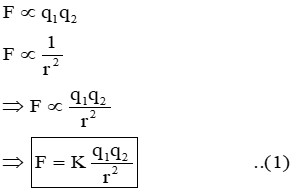
(Where, ‘K’ is a proportionality constant known as ‘Electrostatic force constant’/ ‘Coulomb’s Constant’.) So, equation (1) can also be written as;

![]()
![]()
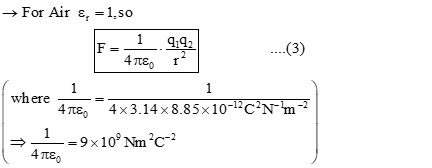
Limitations of Coulomb’s Law
- Coulomb’s law can not be applied if the charges are in motion.
- Coulomb’s law is only applicable for point charges (i.e., the size of the charges must be small as compared to the distance between them.
- The separation between the charges must be greater than 10–15 m. (Because for distances less than 10–15 m, nuclear force dominates over the electrostatic force.)
